Analysis of Hematological and Serum Chemistry Abnormalities in Clinical Trials of Adjunctive Eslicarbazepine Acetate in Children (Aged 4–17 Years) With Focal Seizures
Abstract number :
2.247
Submission category :
7. Antiepileptic Drugs / 7B. Clinical Trials
Year :
2018
Submission ID :
501927
Source :
www.aesnet.org
Presentation date :
12/2/2018 4:04:48 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Prakash Kotagal, Cleveland Clinic Epilepsy Center; Tobias Loddenkemper, Boston Children's Hospital, Harvard Medical School, Boston, MA, USA; Todd Grinnell, Sunovion Pharmaceuticals Inc.; David Cantu, Sunovion Pharmaceuticals Inc.; Yan Li, Sunovion Pharmac
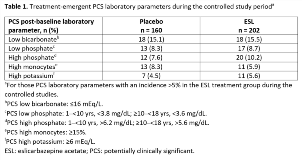
Rationale: Eslicarbazepine acetate (ESL) is a once-daily oral antiepileptic drug (AED) for focal (partial-onset) seizures (FS). Although rare, hematological adverse events have been reported in adults using ESL. Here, we evaluate the occurrence of cytopenia treatment-emergent adverse events (TEAEs) and hematology and serum chemistry abnormalities in clinical trials of ESL for FS in children. Methods: Pooled safety data from subjects aged 4–17 yrs in Studies BIA-2093-208 and -305 were analyzed. Both trials were randomized, double-blind, placebo-controlled studies of adjunctive ESL in children with FS refractory to 1–2 AEDs. Study 208-Part 1 was a 12-week, Phase II study in subjects aged 6–16 yrs, with a target ESL dose of 30 mg/kg/day. Study 305-Part 1 was an 18-week, Phase III study in subjects aged 2–18 yrs, with a target ESL dose of 20 mg/kg/day. Subjects could continue into a 1-yr uncontrolled, open-label extension (OLE) (Part 2), and into further OLEs (Study 208, Part 3; Study 305, Parts 3–5). Incidences of cytopenia medically significant events (MSEs) were evaluated; they included investigator-reported TEAEs in the categories of ‘anemias not elsewhere classified (NEC)’, ‘marrow depression and hypoplastic anemias’, ‘leukopenias NEC’, or ‘neutropenias’ that were fatal, serious, severe, led to discontinuation, or had other action taken. Laboratory samples collected throughout Studies 208 (Parts 1–2) and 305 (Parts 1–5) were evaluated for hematology and serum chemistry abnormalities. Results: The safety population (subjects aged 4–17 yrs who received =1 dose of study drug) for the controlled study period included 362 subjects (ESL, n = 202; placebo, n = 160); 337 continued into the OLE. Cytopenia MSEs: four subjects (2.0%) taking ESL (neutropenia, 1.5%; anemia, 0.5%) and none taking placebo experienced a cytopenia MSE during the controlled studies. Three subjects experienced a cytopenia MSE during the uncontrolled OLEs (pancytopenia: n = 2; anemia: n = 1). Hematology and serum chemistry: there were no clinically significant changes between baseline and highest/lowest on-treatment values for any hematology or serum chemistry parameter during the controlled studies or OLEs. Bicarbonate levels shifted from ‘normal’ at baseline to ‘low’ at some point during the controlled studies in 29.8% of subjects taking ESL and 25.6% taking placebo. Some treatment-emergent potentially clinically significant (PCS) laboratory parameters occurred in >5% of subjects taking ESL (Table 1); all occurred in a similar proportion of subjects taking ESL versus placebo, and in the controlled versus uncontrolled periods. Conclusions: Cytopenia MSEs occurred infrequently during controlled and uncontrolled use of adjunctive ESL for FS in children aged 4–17 yrs. In addition, there were no clinically significant changes between baseline and highest/lowest on-treatment values for any hematology or serum chemistry parameters. Funding: Studies sponsored by BIAL; analyses funded by Sunovion Pharmaceuticals Inc.