Early epilepsy surgery in Tuberous Sclerosis Complex: Preliminary results on safety, efficacy and neurodevelopmental outcomes
Abstract number :
3.456
Submission category :
9. Surgery / 9B. Pediatrics
Year :
2018
Submission ID :
555023
Source :
www.aesnet.org
Presentation date :
12/3/2018 1:55:12 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Jurriaan M. Peters, Harvard Medical School, Boston Children's Hospital; Leslie Grayson, University of Alabama at Birmingham; Darcy Krueger, Cincinnati Children's Hospital Medical Center; Mustafa Sahin, Boston Children's Hospital; Joyce Wu, UCLA Mattel Chi
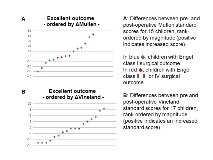
Rationale: Medically refractory seizures in infants with Tuberous Sclerosis Complex (TSC) have a detrimental impact on neurodevelopment, and early surgery should be considered. We determined safety, surgical outcome, and neurodevelopmental changes associated with epilepsy surgery in children less than 3 years of age with TSC. Methods: From 160 children enrolled in the Tuberous Sclerosis Complex Autism Center of Excellence Network (TACERN) prospective multicenter observational study of early predictors of epilepsy and autism spectrum disorder, 34 children underwent epilepsy surgery before 3 years of age. We retrieved demographic and clinical data from case record forms, and reviewed electronic medical records for procedural details, complications and surgical outcomes based on Engel classification. Children with an excellent surgical outcome (Engel I) or good surgical outcome (Engel I and II) were compared to those with poor outcomes (Engel III and IV). Pre- and post-operative Mullen and Vineland composite standard scores were recorded prospectively. Results: Surgical data were available in 25 children, 60% female. Median age at onset of epilepsy was 4 months (range 0.25-7.5), and for the surgically targeted seizure type 6 months (range 0.25 -17). The targeted seizure type was focal dyscognitive seizure in all cases. (64%) also had infantile spasms, occurring before the targeted seizure type in 13. Median number of anti-epileptic drugs at the time of presurgical workup was 3 (range, 2-6), and post-operatively, medications were reduced in 5/17 with available data (29%).Median age at surgery was 19 months with a wide range (3.5-35.5). 11 (25%) underwent a single stage open resection, guided by intraoperative electrocorticography. Subdural and depth electrodes were used in 8 (32%), followed by extraoperative monitoring. 3 (12%) had a 3-staged surgery, and 3 (12%) stereoEEG followed by MR-thermography guided laser interstitial thermal therapy in 2, and an open resection in 1.Open resections were tuberectomy in 7 (28%), multiple tuberectomy or tuberectomy with adjacent white matter in 8 (32%), and partial lobectomy in 6 (24%). Surgical complications occurred in 7 (28%): fluid collection, edema or hemorrhage requiring a drain or reoperation (n=3), motor deficit (n=3), infection (n=2), local infarction (n=1) and visual field deficit (n=1) – although some deficits were anticipated. At 6 months, surgical outcome with respect to reduction of targeted seizure type was Engel I in 15 (60%), Engel I or II in 18 (72%), and Engel III or IV in 7 (28%). In an intention-to-treat analysis, seizure freedom at 6 months, regardless of surgical success in stricto sensu, was only present in 13 (52%) due to emergence of new seizure types.In a linear mixed effects model, excellent (or good) surgical outcomes were not associated with any consistent change in pre- and postoperative Mullen and Vineland standard scores, likely reflecting short duration of follow-up and underpowering of our sample. Visualization of changes in these scores suggests a nearly equal distribution of patients with good and poor outcomes, although few patients with surgical failure had improved scores (Figure 1). Conclusions: Infants and young children with TSC can undergo surgery with the same success rates at 6 months as older children in the literature. The complication rate is higher but many were minor, temporary, or anticipated. More data from this cohort, and a prospective observational surgical study, are needed to allow for determination of neurodevelopmental yield and a potential superior surgical approach with regards to complications and success rate. Funding: NIH P20 NS080199 and U01 NS082320