EFFECT OF AGE AND GENDER ON THE PHARMACOKINETICS OF ESLICARBAZEPINE ACETATE
Abstract number :
2.373
Submission category :
Year :
2005
Submission ID :
5680
Source :
www.aesnet.org
Presentation date :
12/3/2005 12:00:00 AM
Published date :
Dec 2, 2005, 06:00 AM
Authors :
Patricio Soares-da-Silva, Luis Almeida, Amilcar Falcao, and Joana Maia
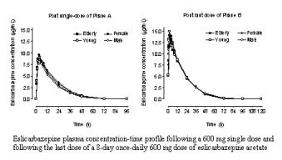
Eslicarbazepine acetate is a novel voltage-gated sodium channel blocker in development for the treatment of epilepsy, bipolar disorder and neuropathic pain. The objective of this study was to determine the effect of age and gender on the pharmacokinetics of eslicarbazepine acetate. Single-centre, open-label, parallel-group study in 12 young (18-40 years) and 12 elderly (65 or over) healthy subjects. In each age group, 6 subjects were female and 6 were male. The study consisted of a single-dose (600 mg) period (Phase A) and a multiple-dose (600 mg, once-daily, for 8 days) period (Phase B), separated by 4 days. Eslicarbazepine acetate was extensively metabolized to eslicarbazepine (S-licarbazepine), the main active metabolite. Plasma concentration-time profiles of eslicarbazepine following the single-dose of Phase A and the last dose of Phase B are presented in Figure 1. Following a 600 mg single-dose, mean maximum eslicarbazepine plasma concentrations (C[sub]max[/sub]) and area under the plasma concentration-time curve from 0 to infinity (AUC[sub]0-[infin][/sub]) were respectively 9.9 [mu]g/mL and 180.9 [mu]g.h/mL in young subjects, and 9.5 [mu]g/mL and 196.0 [mu]g.h/mL in elderly subjects, and respectively 9.3 [mu]g/mL and 171.9 [mu]g.h/mL in male subjects, and 10.1 [mu]g/mL and 205.0 [mu]g.h/mL in female subjects. After multiple-dosing, steady-state plasma concentrations were attained at 4 to 5 days of administration in both age and gender groups, consistent with an effective half-life in the order of 17-18 hoursPost last dose of Phase B, mean C[sub]max[/sub] and AUC[sub]0-[infin][/sub] of eslicarbazepine were respectively 17.3 [mu]g/mL and 296.7 [mu]g.h/mL in young subjects, and 15.1 [mu]g/mL and 294.3 [mu]g.h/mL in elderly subjects, and respectively 15.5 [mu]g/mL and 295.8 [mu]g.h/mL in male subjects, and 16.8 [mu]g/mL and 295.2 [mu]g.h/mL in female subjects. Following single-dose, the eslicarbazepine C[sub]max[/sub], AUC[sub]0-24[/sub] and AUC[sub]0-[/sub][sub][infin][/sub] elderly/young geometric mean ratios (GMR) and their 95% confidence intervals (95%CI) were 0.95 (0.81, 1.14), 1.02 (0.86, 1.24) and 1.06 (0.88, 1.32) and the female/male GMR (95%CI) were 1.09 (0.87, 1.43), 1.16 (0.95, 1.48) and 1.17 (0.90, 1.63), respectively. Following last dose, the eslicarbazepine C[sub]max[/sub], AUC[sub]0-24[/sub], and AUC[sub]0-[/sub][sub][infin][/sub] elderly/young GMR (95%CI) were 0.88 (0.77, 1.03), 0.98 (0.90, 1.09), and 1.01 (0.89, 1.18) and the female/male GMR (95%CI) were 1.10 (0.98, 1.27), 1.04 (0.88, 1.28), and 1.01 (0.83, 1.30), respectively. The pharmacokinetic profile of eslicarbazepine acetate was not affected either by age nor gender.[figure1]