EFFECTIVENESS AND TOLERABILITY OF OXCARBAZEPINE(TRILEPTAL[reg]): RESULTS OF AN OBSERVATIONAL STUDY IN 325 CHINESE PATIENTS
Abstract number :
2.385
Submission category :
Year :
2005
Submission ID :
5692
Source :
www.aesnet.org
Presentation date :
12/3/2005 12:00:00 AM
Published date :
Dec 2, 2005, 06:00 AM
Authors :
Li-Ping Zou, and Li-Wen Wu
[middot] Oxcarbazepine (Trileptal[reg]) was approved by the SFDA in 2004 for the treatment of partial or generalized tonic-clonic epilepsy in children and adults.
[middot] Oxcarbazepine has a different pharmacokinetic profile comparing to carbamazepine. From US and European studies Oxcarbazepine shows an improved safety profile, but there is limited data from Asian populations.
[middot] The recommended dosing of oxcarbazepine is mostly from studies in Caucasians. Information regarding the appropriate dosing in Asian populations is needed . [middot] This was a prospective open-label, study conducted in Chinese patients with partial or generalized tonic-clonic epilepsy who were newly diagnosed.
[middot] Treatment period was 24 weeks that included flexible dosing of oxcarbazepine monotherapy.
[middot] Efficacy assessments were seizure free rate and seizure frequency reduction [ge]50% (percentage of patients).
[middot] Adverse events (AEs) were recorded throughout the study. [middot] A total of 325 patients were enrolled. Patient characteristics are shown in Table 1.
[italic]Responder rate
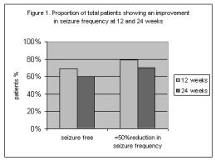
[/italic][middot] Overall, 70% of patients responded to therapy at 24 weeks. Of these patients, 225(69%) and 196( 60%) were seizure free at week 12 and 24 respectively (Figure 1).
[middot] The starting dose of oxcarbazepine was 251.85[plusmn]129.34(50-750)mg/day and the maintenance dose was 644.67[plusmn]287.69(150-1500)mg/day. The is no significant different among different age groups.
[middot] A total of 63(19%) patients experienced AE[apos]s, leading to discontinuation in only 3.1%. The most frequency reported AEs were dizziness 11(3.4%); headache 6(1.8%); somnolence 14(4.3%); asthenia 4 (1.2%) and skin rash 9 (2.8%). [middot]The results from this study of newly diagnosed patients with partial or generalized tonic-clonic epilepsy confirm the efficacy and tolerability of oxcarbazepine, when prescribed as monotherapy for Chinese patients.
[middot]A maintenace dose of oxcarbazepine close to 650mg allows for optimal seizure control with an excellent tolerability profile. This dosage seems to be lower than Caucasians.
[middot]The nature, frequency and severity of AEs reported in this study were consistent with those previously reported in US or European studies.[figure1][table1] (Supported by Beijing Novartis Pharma Ltd.)