Healthcare Resource Utilization and Cost Before and After Adding Lacosamide as Adjunctive Therapy Among Patients Diagnosed With Epilepsy: A Retrospective U.S. Claims Analysis
Abstract number :
2.258
Submission category :
7. Antiepileptic Drugs / 7C. Cohort Studies
Year :
2018
Submission ID :
481172
Source :
www.aesnet.org
Presentation date :
12/2/2018 4:04:48 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
David M. Labiner, University of Arizona; Barbara Johnson, Truven Health Analytics; Melinda Martin, UCB Pharma; Jesse Fishman, UCB Pharma; and Carolyn Lew, Truven Health Analytics
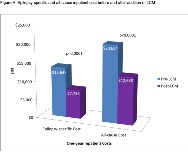
Rationale: In placebo controlled pivotal trials in partial-onset seizures, patients who were not adequately controlled with 1 to 3 concomitant AEDs who used lacosamide (LCM) as an adjuvant therapy typically had 35-40% fewer seizures compared with their initial treatment. This retrospective database analysis compared epilepsy-related and all-cause healthcare resource utilization (HRU) and costs in a real world setting among patients with epilepsy in the U.S. before and after initiating LCM as adjunctive therapy for treatment of focal (partial-onset), generalized, and other seizures. Methods: Patients diagnosed with epilepsy who added LCM to existing mono-or poly- antiepileptic drug (AED) therapy between 2009-2016 (index event) were identified in the IBM MarketScan® databases. Patients were required to have a minimum of 12 months continuous eligibility before (pre-LCM period) and after (post-LCM period) their index event. In the 12-month post-LCM period, the only allowed regimen change was addition of LCM. Demographic and clinical characteristics were measured at index and during the pre-LCM period, respectively. All-cause and epilepsy-specific HRU and costs were measured in the pre- and post- LCM periods. Paired t- and McNemar's tests were conducted to assess the significance of differences pre-LCM and post-LCM. Results: The study sample comprised 2,171 patients mean (standard deviation [SD]) age 38.9 (19.3) years; 52.6% female. Just over half (56%) were on monotherapy prior to adding LCM. The most common CNS-related comorbidities were anxiety (15.4%), headache (14.0%), depression (13.0%), and cerebrovascular disease (12.2%). The top 3 AEDs to which LCM was added were levetiracetam (54.4%), lamotrigine (24.9%) and topiramate (16.1%). Prior to adding LCM, 28.8% of patients had an epilepsy-specific inpatient (IP) admission and 35.7% of patients had an all-cause IP admission compared to 18.2% and 26.1% of patients in the post-LCM period, respectively (both P<0.0001). Likewise, 35.6% of patients had an epilepsy-specific emergency room (ER) visit and 50.0% had an all-cause ER visit prior to adding LCM, compared to 23.8% and 42.1% following addition of LCM, respectively (both P<0.0001). One-year mean (SD) epilepsy-specific and all-cause inpatient costs decreased from $13,647 ($52,590) to $7,788 ($32,321) and from $20,654 ($72,716) to $12,688 ($46,120) after adding LCM (both P<0.0001). One-year epilepsy-specific and all-cause ER costs decreased from $691 ($1,756) to $448 ($1,909) (P<0.0001) and from $1,217 ($3,014) to $1,000 ($2,970) (P=0.002) following addition of LCM. Conclusions: Indicators of seizures, epilepsy-related inpatient hospitalizations and ER visits, were significantly reduced in patients with epilepsy 12 months after adding LCM as adjunctive therapy to existing AED treatment in a real-world setting, leading to reduced healthcare resource utilization and epilepsy costs. Funding: UCB Pharma-sponsored
.tmb-.png?Culture=en&sfvrsn=e7dc5e6f_0)