Minimally Invasive Surgical Approach for Subdural Electrode Implantation in Focal Epilepsy
Abstract number :
1.088
Submission category :
2. Translational Research / 2B. Devices, Technologies, Stem Cells
Year :
2018
Submission ID :
500936
Source :
www.aesnet.org
Presentation date :
12/1/2018 6:00:00 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Mark Benscoter, Mayo Clinic; Sanjeet S. Grewal, Mayo Clinic; Stephen Kuehn, Mayo Clinic; Joel Kuhlmann, Mayo Clinic; Seth Hara, Mayo Clinic; Gregory A. Worrell; and Jamie J. Van Gompel
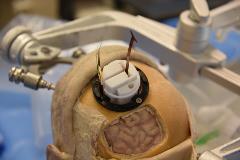
Rationale: Focal drug resistant epilepsy affects approximately 3 million patients each year in the U.S. and approximately 60 million worldwide. Approximately 30% of people that are diagnosed with focal epilepsy are drug resistant and may benefit from surgical treatment. These patients may be eligible for alternative therapeutic procedures that include placement of subdural electrodes for electrical stimulation or surgical tissue resection. The use of subdural grid electrodes currently requires larger craniotomies than expected seizure onset zone leading to complications in 8% of patients. An important opportunity exists to develop an innovative approach to obtain subdural access using a greatly reduced craniotomy size and also provide a method to introduce subdural electrodes with high placement accuracy. Methods: We developed a novel electrode delivery appliance for delivering electrodes into the subdural space assisted by flexible endoscopy. The effectiveness of this approach was tested by developing a 3D printed anatomical model of the skull and brain based on human CT data, and by conducting cadaveric studies using fresh specimens. Multiple tests were conducted using the 3D printed skull and brain model, and six cadaver tests were conducted. The results of the testing on the 3D printed model were used to refine the device design prior to conducting the cadaver testing. The results of the cadaver testing were used to influence biocompatible device design requirements and surgical technique refinement. Results: An approximately 3.5 cm2 keyhole craniotomy, our delivery appliance, wedge-shaped thin film electrode configurations, commercially available electrode arrays, and proof of concept electrode designs were all used to achieve broad ranging cortical surface coverage. Using this innovative electrode delivery appliance, the average size of the craniotomy was greatly reduced when compared to the traditional craniotomy. The device enabled commercialized linear electrode strips, as well as, proof of concept electrode designs to be delivered into the subdural space. Finally, electrode delivery is able to be visualized in the intracranial space to validate electrode placement. Conclusions: This new surgical approach with a reduced craniotomy size would greatly reduce post procedure patient monitoring risk and provide accurate EEG data. Additionally, this approach provides surgical tools that enable electrode placement accuracy and greater placement location options. Finally, the improved accuracy of electrode placement alters patient epileptic monitoring for more precise subsequent therapeutic planning. Funding: This effort was in part funded through Mayo Clinic internal funding mechanisms.
.tmb-.jpg?Culture=en&sfvrsn=7a05e80c_0)