Predicting Early Study Drop-Outs Using Adherence Data from a Mobile Daily Diary Tracking Device
Abstract number :
3.338
Submission category :
Late Breakers
Year :
2013
Submission ID :
1867053
Source :
www.aesnet.org
Presentation date :
12/7/2013 12:00:00 AM
Published date :
Dec 5, 2013, 06:00 AM
Authors :
L. Ernst, C. Lau, P. Pennell, J. French, R. Kashambwa, N. Llewellyn, K. Kniseley, B. Kaufman, E. Bartfeld, C. Harden
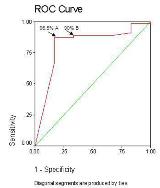
Rationale: WEPOD is a multicenter prospective observational study evaluating fertility among women with epilepsy (WWE) compared to healthy controls. WEPOD utilizes a customized mobile electronic patient diary on a commercially available mobile device (Apple s iPod Touch) . A customized diary application made by Irody tracks fertility information, and in WWE, seizures and daily medication use. The app delivers all patient captured information to a cloud database where it is analyzed. We determined the degree of data tracking adherence associated with dropping out of the study.Methods: WWE planning pregnancy enrolled within 6 months of stopping birth control. Subjects were trained to use a customized application (the WEPOD App ) for daily data tracking. All subjects were asked to track data daily until delivery or up to 12 months if they did not become pregnant. Data were analyzed by percent of days in the study for which data was tracked. The cut point of percent tracking with the maximum sensitivity and specificity associating with dropping out of the study was determined. Further, the timing of dropping out of the study was evaluated. Results: Of 61 WWE who enrolled before 12/1/12 and tracked data, 9 subjects dropped out and 52 continued in the study. The overall adherence was 92.3%; 36/61 had 100% adherence. Mean percent adherence of the 52 completed subjects was 96.1% (median 100, range 33-100). Of the 9 subjects who dropped out, percent adherence was 6, 22, 55, 77, 86, 87, 90, 100 and 100 for a mean of 69.9 (median 86). Only 4 subjects were less than 90% tracking adherent and completed the study. The optimal percent adherence cut point for associating with dropping out of the study was 96.5% which had a sensitivity of 87% and a specificity of 83% (point A). Given that the cut point of 96.5 % adherence is higher than the overall mean adherence, and that specificity may be less important than sensitivity since other factors accounting for late drop outs are not accounted for, a more reasonable cut point may be at a sensitivity of 89% and specificity of 67%, which occurred at a tracking adherence of 90% (point B). Subjects who dropped out and were less than 90% tracking adherent did so after a mean of 68 days in the study (median 71 days, range 45-96). Three subjects were more than 90% adherent and did not complete the study; these subjects dropped out at 14, 29 and 234 days of the study. Conclusions: Less than 90% adherence with tracking daily data may be a useful measure for predicting early drop-outs in a prospective study. Further, the subjects with less than 90% adherence dropped out within three months of starting the study. This is a period consistent with the prospective baseline of many interventional trials. Since percent adherence is easily determined using electronic data capture devices, this information may be useful at the onset of clinical studies for predicting subject retention or for identifying subjects who need vigilant monitoring.