Relationship Between Adverse Event Incidence and Eslicarbazepine Acetate (ESL) Dose Across Weight Groups in Clinical Trials of Adjunctive ESL in Children (Aged 4–17 Years) With Focal Seizures
Abstract number :
2.239
Submission category :
7. Antiepileptic Drugs / 7B. Clinical Trials
Year :
2018
Submission ID :
501731
Source :
www.aesnet.org
Presentation date :
12/2/2018 4:04:48 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Jesus E. Piña-Garza, Tristar Medical Group Neurology; Steven Wolf, Icahn School of Medicine at Mount Sinai; Patricia McGoldrick, Icahn School of Medicine at Mount Sinai; Todd Grinnell, Sunovion Pharmaceuticals Inc.; David Cantu, Sunovion Pharmaceutic
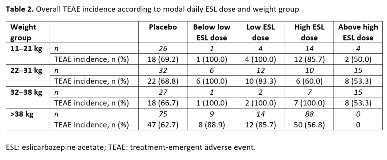
Rationale: Eslicarbazepine acetate (ESL) is a once-daily oral antiepileptic drug (AED) for focal (partial-onset) seizures (FS). Here, we evaluate the occurrence of treatment-emergent adverse events (TEAEs) in clinical trials of ESL for FS in children, according to ESL dose across weight groups. Methods: Pooled safety data from subjects aged 4–17 years in Studies BIA-2093-208 and -305 were analyzed. Both trials were randomized, double-blind, placebo-controlled studies of adjunctive ESL in children with FS refractory to treatment with 1–2 AEDs. Study 208-Part 1 was a 12-week, Phase II study in subjects aged 6–16 years, with a target ESL dose of 30 mg/kg/day. Study 305-Part 1 was an 18-week, Phase III study in subjects aged 2–18 years, with a target ESL dose of 20 mg/kg/day. Incidences of investigator-reported TEAEs were evaluated according to modal daily ESL dose category (below low, low, high, and above high) and weight group (11–21 kg, 22–31 kg, 32–38 kg, and >38 kg) (Table 1). Results: The safety population (subjects aged 4–17 years who received =1 dose of study drug) included 362 subjects (ESL, n = 202; placebo, n = 160), with most subjects in the 7–11 (41%) and 12–17- (44%) years age groups (median age: ESL, 11; placebo, 10). The overall incidence of TEAEs was generally higher with ESL versus placebo, but there was no dose response in any of the weight groups (Table 2). In addition, there was no dose response for the six most frequently reported TEAEs (headache, somnolence, vomiting, nasopharyngitis, pyrexia, and partial seizures), in any weight group. Furthermore, the overall incidence of TEAEs (Table 2), as well as incidences of the most frequently reported TEAEs, did not appear to vary with subject weight in any modal dosing category. Conclusions: When assessing tolerability according to modal daily ESL dose category in four groups defined by subject weight, neither overall TEAE incidence, nor incidences of the most common individual TEAEs, increased with increasing modal daily ESL dose in clinical trials of ESL for FS in children aged 4–17 years. Funding: Studies sponsored by BIAL; analyses funded by Sunovion Pharmaceuticals Inc.
.tmb-.png?Culture=en&sfvrsn=3023b386_0)