SEIZURE FREEDOM IN NEWLY DIAGNOSED PATIENTS WITH PARTIAL SEIZURES RECEIVING OXCARBAZEPINE AS MONOTHERAPY
Abstract number :
2.374
Submission category :
Year :
2005
Submission ID :
5681
Source :
www.aesnet.org
Presentation date :
12/3/2005 12:00:00 AM
Published date :
Dec 2, 2005, 06:00 AM
Authors :
Monique Somogyi, and Kevin McCague
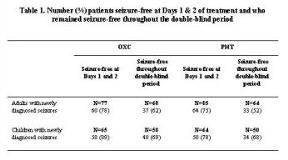
Seizure freedom is the primary goal in the treatment of patients with epilepsy over the long term. Seizure freedom 1-2 days after initiation of monotherapy with oxcarbazepine (OXC) in patients with partial seizures remaining seizure free throughout 56 weeks of treatment was assessed in a subanalysis of two clinical trials. The efficacy and safety of monotherapy with OXC compared with phenytoin (PHT) in adults (16-63 years) (Bill et al. Epilepsy Res 1997) and children (5-18 years) (Guerreiro et al. Epilepsy Res 1997) with newly diagnosed seizures was evaluated in two multicenter, double-blind, randomized, parallel-group clinical trials. During a 8-week titration period in both studies, OXC was initiated at 300 mg/day (adults) and 150 mg/day (children) and PHT at 100 mg/day (adults) and 50 mg/day (children) and titrated to a maximum dose of OXC 2400 mg/day and 800 mg/day PHT. After the titration periods, patients were maintained on their maximum tolerated dose during a 48-week maintenance period. Seizure freedom rates for patients with seizures at baseline who were seizure free at days 1 and 2 of treatment and remained seizure free for the duration of double-blind treatment were assessed. Adverse events (AEs) for this subset of patients were also tabulated. For adults seizure free at Days 1 [amp] 2 who remained seizure free throughout the double-blind period, the mean age was 29 years for both groups, and the baseline partial seizure rate/28 days was 1.5 and 2.9 for the OXC and PHT groups, respectively. For the same subset of children, the mean age was 10 and 11 years and the baseline partial seizure rate/28 days was 1.8 and 2.7 for the OXC and PHT groups, respectively. Seizure freedom rates are presented in Table 1.[figure1]In adults, 1 (3%) and 2 (6%) of OXC- and PHT-treated patients, respectively, discontinued due to AEs. One PHT-treated patient died during the study. The most common ([ge]20% of patients) AEs in the OXC-treated patients were headache, somnolence, viral infection, dizziness, nausea, and vomiting. In children, 1 (3%) and 3 (9%) of OXC- and PHT-treated patients, respectively, discontinued due to AEs. The most common ([ge]20% of patients) AEs in the OXC-treated patients were headache, somnolence, viral infection, and fever. The majority of adults and children with newly diagnosed partial seizures initiated with OXC monotherapy and seizure free at Days 1 [amp] 2 of treatment remained seizure free for more than 1 year. Overall, OXC was safe and well tolerated. (Supported by Novartis Pharmaceuticals.)