Successful Treatment of Refractory NMDA Antibody Encephalitis With Intrathecal Rituximab
Abstract number :
3.435
Submission category :
18. Case Studies
Year :
2018
Submission ID :
502151
Source :
www.aesnet.org
Presentation date :
12/3/2018 1:55:12 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Maritsa Casares, Florida Hospital, Orlando; Holly J. Skinner, Florida Hospital, Orlando; Elakkat D.Gireesh, Florida Hospital, Orlando; Chistina M. Wombles, Florida Hospital, Orlando; Josephine Schweitzer, Florida Hospital, Orlando; Patricia G. Gwyn, Flori
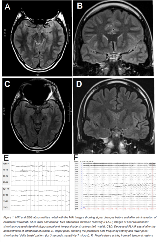
Rationale: NMDA antibody (Ab) encephalitis results from antibodies attacking NMDA receptors in the brain, leading to neuropsychological symptoms. Most common in young women, it often associated with ovarian teratomas. Treatments include immune modulating agents and removal of teratomas. Only 50% of patients recover with 1st line therapy. Patients refractory to these treatments have been treated with 2nd line therapies including intravenous (IV) rituximab. Moreover, 25% of patients may have severe deficits or fatal outcomes.4 Poor penetration of rituximab into CSF could lead to incomplete removal of NMDA Ab. In this report, we will report on a woman with refractory NMDA Ab encephalitis, who was treated with intrathecal rituximab. Methods: A 20-year-old female presented with headache, agitation, then aphasia, inability to follow commands and episodes of body stiffening. Complete paraneoplastic workup was ordered. Brain MRI demonstrated increased T2 and FLAIR signal in the left greater than right hippocampi (figure 1A and B). Imaging of the chest, abdomen and pelvis were negative. EEG showed a delta brush pattern with diffuse slow waves and superimposed beta (figure 1E). Initial treatment were IV corticosteroids and IVIG, which were started before NMDA Ab results were known. Still, the patient was aphasic, not following commands, had orofacial dyskinesias and developed left temporal seizures (figure 1F). Serum NMDA Ab titer was 1:160, so IV rituximab was given. With no clinical improvement, intrathecal rituximab 25 mg weekly for 4 weeks was used. Serum NMDA Ab levels remained at 1:10 during treatment. Results: Two days after the 1st dose of intrathecal rituximab, the patient was following commands. Orofacial dyskinesias and agitation were markedly reduced. One month later, hippocampal hyperintensity on MRI brain decreased compared to pretreatment (figure 1 C and D). Day 49, after a brief rehabilitation unit stay, she was sent home on oxcarbazepine and prednisone for one month. At 2 months, seizures and dyskinesias had resolved. Neuropsychologic evaluation at 3.5 months estimated moderate decline in cognitive abilities relative to her estimated premorbid average to high level. Her level of verbal ability fell in the low average range. As these evaluations were done early in the recovery process, additional improvements are expected. Conclusions: Treatment outcomes have been correlated with severity of symptoms and time from symptom onset to treatment. In a cohort study by Titulaer et. al. refractory NMDA Ab encephalitis patients who received rituximab experienced improved outcomes compared to only first-line immunotherapy. The proposed mechanism of rituximab targets CD20 B-cells and leads to cell lysis. IV rituximab penetration into the CSF is 0.1% of serum. Differences between IV and intrathecal rituximab, could be explained by improved removal of CSF antibodies. We suggest that our patient recovered due use of intrathecal rituximab. We cannot rule out that delayed effects of IV rituximab and/or concurrent use of steroids that may have played a role in her recovery. Funding: There are no financial sources.